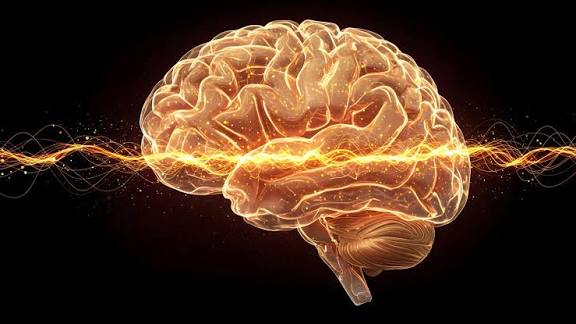
Stopping Alzheimer’s may begin with dissolving tiny tau protein clusters before damage takes hold.
Scientists at Tokyo Metropolitan University have turned to polymer physics to better understand one of the defining features of Alzheimer’s disease: the formation of tau protein fibrils. Their research shows that these fibrils do not form directly. Instead, tau proteins first gather into large clusters, similar to how polymers begin to crystallize. When researchers disrupted these early clusters, fibrils failed to develop in solution.
This finding points to a major shift in how future treatments for neurodegenerative diseases might be designed.
Why Alzheimer’s Remains So Difficult to Treat
Alzheimer’s disease (AD) remains one of the most complex and challenging disorders facing researchers today. Understanding how it progresses and finding effective treatments have proven difficult, especially as aging populations worldwide increase the number of people affected.
While much of the research has focused on pharmacology and traditional medical approaches, the intricate nature of AD has pushed scientists to draw on ideas from outside disciplines to uncover new perspectives and solutions.
Using Polymer Crystallization to Understand Tau Fibrils
A research group led by Professor Rei Kurita applied principles from polymer physics to study how tau protein fibrils form in AD. Polymers, which are long chain-like molecules, often organize themselves into crystals through a multi-step process. Rather than growing crystal structures one strand at a time, polymers frequently pass through intermediate stages known as “precursor” structures before settling into an ordered form.